
Our research is mainly focused on Micro/nanoscale flows, Soft Matter, and Biofluidics with a strong focus on physics. Our studies elucidate several intriguing advanced physical phenomena through a synergy of experiment, theory, and simulations. These studies lead to significant advancements to existing knowledge in terms of new experimental protocols, unique observations, new theoretical formulation, and critical physical insights in physics hitherto unexplored. Our studies involve flows inside confined geometries such as microchannels and microcavities, involving particles, drops, and bubbles, with varying viscous and elastic properties, relying on the inherent hydrodynamics or external fields such as ultrasound and magnetic fields. Currently, we mainly focus on studying the Micro/nanoscale flows and Soft Matter physics in the emerging ultrasound-microfluidics or acousto-microfluidics framework. The advancements in physics in several research problems have spurred the development of microfluidics technology for diagnostics, and healthcare applications, as well as space applications.
Our work is targetted towards developing the following microfluidics technologies: (1) In vitro Diagnostics, (ii) Therapeutics (iii) Assisted Reproductive Technology, (iv) Space technology.
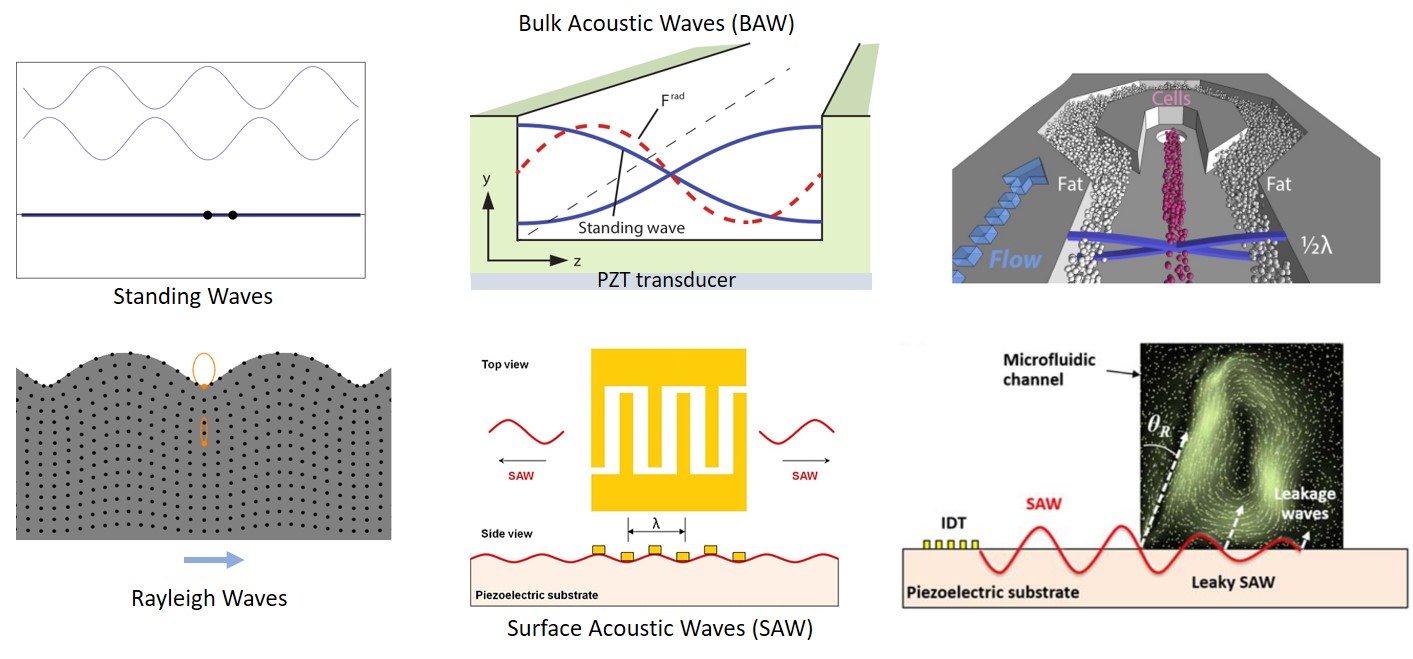
Acousto-microfluidics which enables handling of particles and fluid interfaces using ultrasonic waves has emerged as a power tool in the recent past due to its inherent nature of gentle, precise and contactless manipulation. Two types of waves, namely bulk and surface acoustic waves can be employed to perform manipulations. Bulk acoustic waves (BAW) can be generated using piezoelectric transducers. Bulk acoustic standing waves can be produced in a channel using a piezoelectric actuator bonded to the device and by adjusting the frequency of the wave generated with respect to the channel dimensions. Surface Acoustic waves (SAW) can be generated using an array of interdigitated electrodes patterned on a piezoelectric substrate at an appropriate frequency related to the gap between the electrodes. Both travelling surface acoustic waves (TSAW) and standing surface acoustic waves (SSAW) can be employed for the manipulation of particles and fluid interfaces.
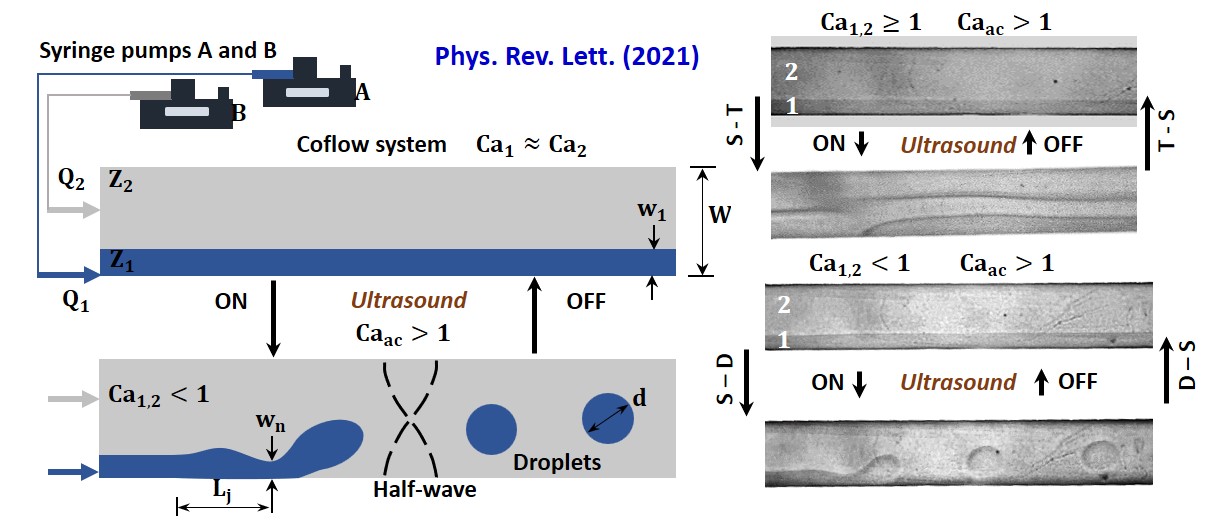
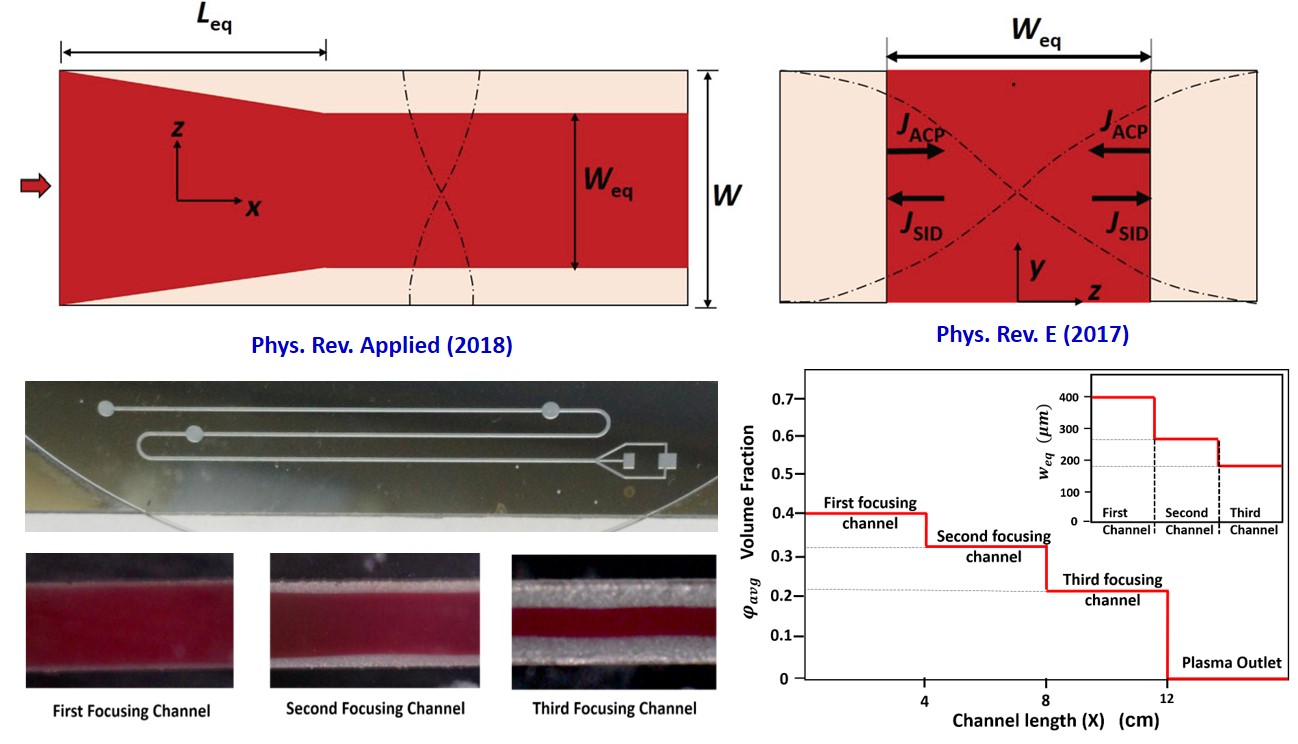
Transition between stream and droplet regimes in a coflow is typically achieved by adjusting the capillary numbers (Ca) of the phases. Remarkably, we experimentally evidence a reversible transition between the two regimes by controlling exposure of the system to acoustic standing waves, with Ca fixed. By satisfying the ratio of acoustic radiation force to the interfacial tension force, Ca_ac>1, experiments reveal a reversible stream-drop transition for Ca<1, and stream relocation for Ca≥1. We explain the phenomenon in terms of the pinching, advection, and relocation timescales and a transition between convective and absolute instability from a linear stability analysis. The technique offers means of tuning from stream to droplet mode on-demand, which can find applications in microfluidics, emulsification, and encapsulation.
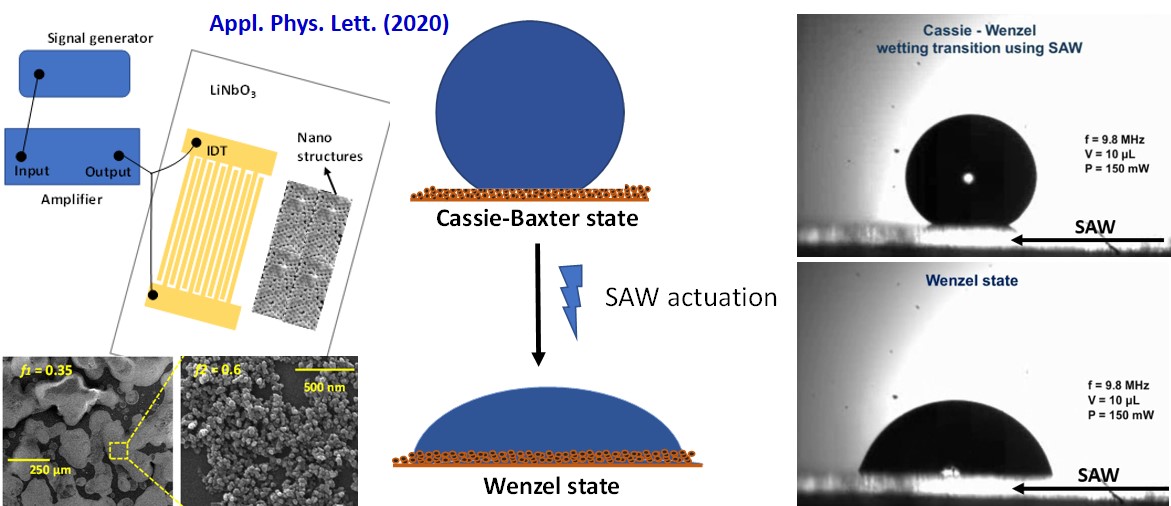
We investigated irreversible Cassie-Wenzel (C-W) transition on a nanostructured superhydrophobic surface employing surface acoustic wave (SAW) vibration. The transition is achieved upon penetration of the liquid into the nanogrooves driven by the inertial energy of the drop imparted by the SAW. However, the filling up of nanopores imposes energy barrier (Eb) to the transition that requires the displacement of the initial solid-air interface inside the pores with a solid-liquid interface. We unravel that the relative magnitudes of the input acoustic energy (Eac) and this energy barrier dictates the wetting transition; the irreversibility in the transition therefore being explained from energy minimization of the system following the transition. In addition, observing the dynamics of the wetting front allowed the different regimes of the wetting transition process to be identified.
.jpg)
We have been focusing on the development of capillaryry based power-free microfluidic chips integrated with optical biosensors for rapid but quantitative detection/measurement of blood based analytes. For instance, we have been developing a biosensor that exploits the localized surface plasmon resonance (LSPR) effect of nanostructures to detect dengue NS1 antigen. The biosensor integrates a blood-plasma separation to develop lab-on-chip device that facilitates rapid diagnosis (within 30 min) of dengue NS1 antigen from a small volume of whole blood. From 10 µL of whole blood spiked with NS1 antigen, our biosensor reliably detects 0.06 µg/mL of NS1, which lies within the clinical limit observed during the first seven days of infection, with a sensitivity of 9 nm/(µg/mL). These results confirm that the proposed LSPR biosensor can potentially be used in point-of-care dengue diagnostics.

We have been developing a generic microfluidic platform for the detection or measurement of blood-based analytes. The platform constitutes a plasma separation unit to fractionate blood into cells and cell-free plasma, a mixing & reaction unit wherein the cell-free plasma is mixed and reacted with a suitable chemical probe/antibody producing a fluorophore which is then measured/detected in an optical detection unit for fluorescence/absorbance or other optical signals. As an example, we have been developing a microfluidic platform for early detection of sepsis. Apart from the routine biomarkers such as Lactate, and Procalcitonin, we plan to improve risk prediction via the imbalance of gaseous signaling molecules - gasotransmitters that are intimately related to cellular processes. We have developed a microfluidic device that utilizes fluorimetry for the continuous monitoring of the concentration of different gasotransmitters such as H2S, H2O2 , NO and CO in patients’ blood. The plan is to use the gasotransmitter profiles along with the existing clinical parameters to improve risk prediction and prognosis in sepsis.

Spheroids are compact, spherical aggregations of cells that can be used to model tumor tissues in a laboratory setting. Spheroids are created by injecting cells into a specialized microenvironment, where they form cell-to-cell interactions and compact into a spherical structure. Spheroids are used in drug screening and testing because they more closely resemble in vivo tumor tissues than two-dimensional cell cultures. We are currently working on the high-throughput fabrication of 3D tumor models (cancer spheroids) for drug screening. Such 3D models will also be used for studying their interactions with immune cells and CAR-T cells.

Microbubbles are small gas-filled bubbles that have many applications in medicine, industry, life science, and food technology. Microbubbles can be used to carry drugs to specific areas of the body. They are often designed to preserve the drug until it reaches the desired area, and then ultrasound is used to disintegrate the microbubbles and release the drug. Microbubbles are a new treatment modality for cancer. Microrobots, which are easy to break through various barriers and have stronger controllable locomotion ability that can realize active transport of drugs, have attracted extensive attention in drug release. We are developing tools for the controlled transport of microbubbles through micro-robotics for controlled drug delivery applications.

Circulating tumor cells (CTCs) occur in very low frequency in whole blood, i.e. 1 mL of human blood could contain 1-100 CTCs among a few million white blood cells (WBCs) and a billion red blood cells (RBCs). Quantification of CTCs has high clinical value as these cells usually indicate tumor metastasis and facilitate real-time monitoring of systemic therapy by sequential peripheral blood sampling. Molecular characterization of CTCs helps to understand the mechanism of metastasis, enables the identification of therapeutic targets, and contributes to personalized, anti-metastatic therapies.

India possesses more than 300 millions of bovine population, both cattle and buffalo. Natural breeding or artificial insemination provides equal chance of birth of a female or a male calf. Adoption of mechanization in agriculture and industry has reduced utility of male cattle and buffalo. Thus, there is need to have more number of female cattle and buffaloes for milk and milk products while limited number of males are required for breeding and other farm activities. Sexed-semen-based artificial insemination technology is a promising technique to bring rapid and sustainable improvement in dairy cattle breeding. Bovine sperm sorting is used to increase the number of female calves in dairy herds, as female calves are more valuable for milk production and genetic advancement. Artificial insemination with sorted sperm to be a promising way to increase agricultural efficiency.


We have been working with ISRO to develop a microthruster for manuevering micro/nano/pico satellites that weigh between 100s of grams to a few killograms, mostly for inspection of spacecrafts. Compared to chemical and electric microthrusters, the electrospray microthrusters offer high-specific impulse without compromising the thrust requirements. We have also been developing a micropump that can supply propellants to the microthrusters. Ultrasonic micropumps do not involve any moving parts and can function well in microgravity scenarios. The micrompus will also find applications in biomedical engineering.